ACR _ Introduction
Asia CML Registry (ACR)

What is ACR?
Asia CML Registry (ACR) is a web-based registry system for chronic myeloid leukemia patient. Its web-based property provides convenient access to ACR. You can access the system whenever you want and wherever you are.
However, ACR is not a simple registry. Actually it is portal database system. In ACR you can input patient data in more than 50 different fields, which actually cover clinical and demographic record fields including CBC, cytogenetics, BCR-ABL transcript level, mutation, drug treatment, and hematopoietic stem cell transplantation.
Above all, one of the advantages of ACR might be its excellent management function for patient data. You can investigate sequential clinical data of a single patient. In addition, yon can extract patient group and their data with specific features by filtering system.
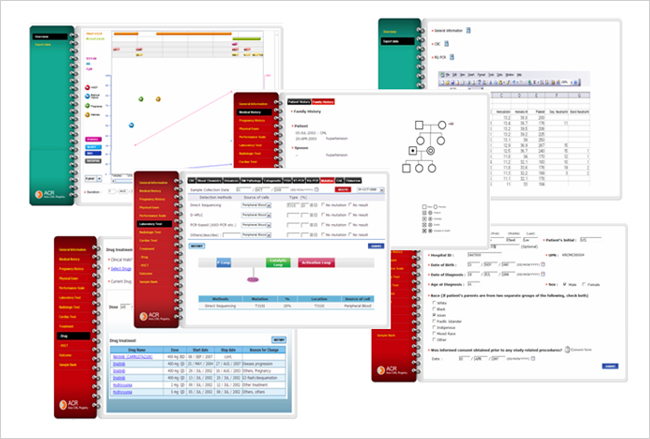
You can input patient demographic data and clinical data using user friendly input system, and even you can input medical history of patient's family.
All clinical laboratory test results can be recorded and then maintained in a sequential analytical manner. All treatments can be recorded systematically with other information.
Major stored information of a patient can be observed in graphic manner, which is very convenient for overview of responses of the patient.
Policy of ACR
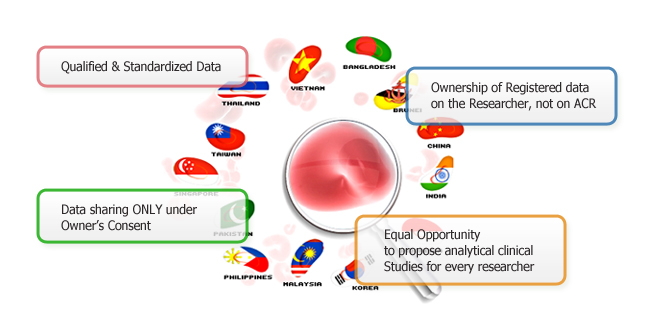
For excellent management of patient clinical data, ACR will add only the qualified and standardized clinical data in ACR database system. ASMA (Asia Standardization of Molecular Assay) as a part of ACSA will provide molecular assay training for ACSA members who want to learn standardized molecular technologies.
Even all data are stored and maintained safely in ACR server, ownership of input data is on the research, not on ACR. Without owner's consent, ACR can not use the owner's data for any purpose.
When analytical clinical study is proposed, ACR will recruit the researchers who want to participate in the study. In this case, data of the participated researchers will be shared for analytical purpose under the agreement of data owner.
Equal opportunity will be given to propose analytical clinical studies for every researcher who registered patients' clinical data. The opportunity will never be overwhelmed by the number of patients each researcher registered in ACR.
How does ACR work?
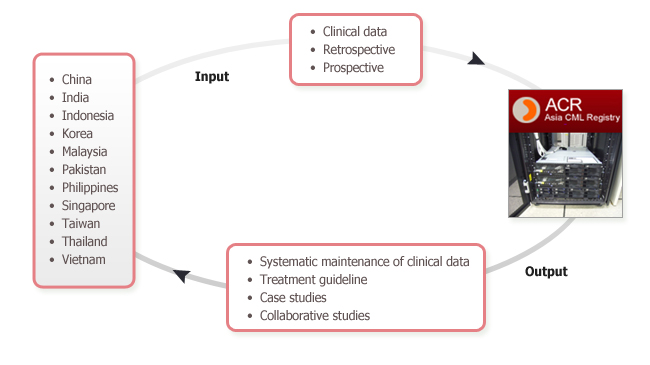
Each site in Asian region will input CML patients' clinical data retrospectively or prospectively. The input data will be stored safely in ACR server located in Molecular Genetics Research Institute, The Catholic University of Korea, Seoul, South Korea.
Each site will get benefits including systematic maintenance of their clinical data, clinical information (treatment guideline and case studies). In addition, with their input data, each site can participate in analytical clinical studies, and will be author when ACSA (Asia CML Study Alliance) publish the analytical results in international journals.
Other information
For ACR, you need to have your registration account. Application to ACR account can be made to ACSA office.
ACR will be report current registration status of each site and any interesting update of system twice a year in ACSA meeting. First annual ACSA meeting will be held during EHA (European Hematology Association) congress in June, and second annual ACSA meeting will be held during ASH (American Society of Hematology) annual meeting in December.












